This lesson focuses on the identification of aluminum, exploring the case of madder lake. Aluminum, with an atomic number of 13, can be detected by its K-alpha line at 1.5 keV. Understanding how aluminum is used in madder lake formulations helps in analyzing historical and modern recepes for this and other lakes. The lesson explores different types of madder lake and their elemental compositions to determine the role of aluminum and other elements used as mordants.
Objectives









- Identify the presence of aluminum in madder lake.
- Compare aluminum signals in different reference materials, such as pure aluminum and alum.
- Analyze variations in madder lake compositions based on different mordants.
- Pure aluminum reference sample
- Alum (potassium aluminum sulfate) reference sample
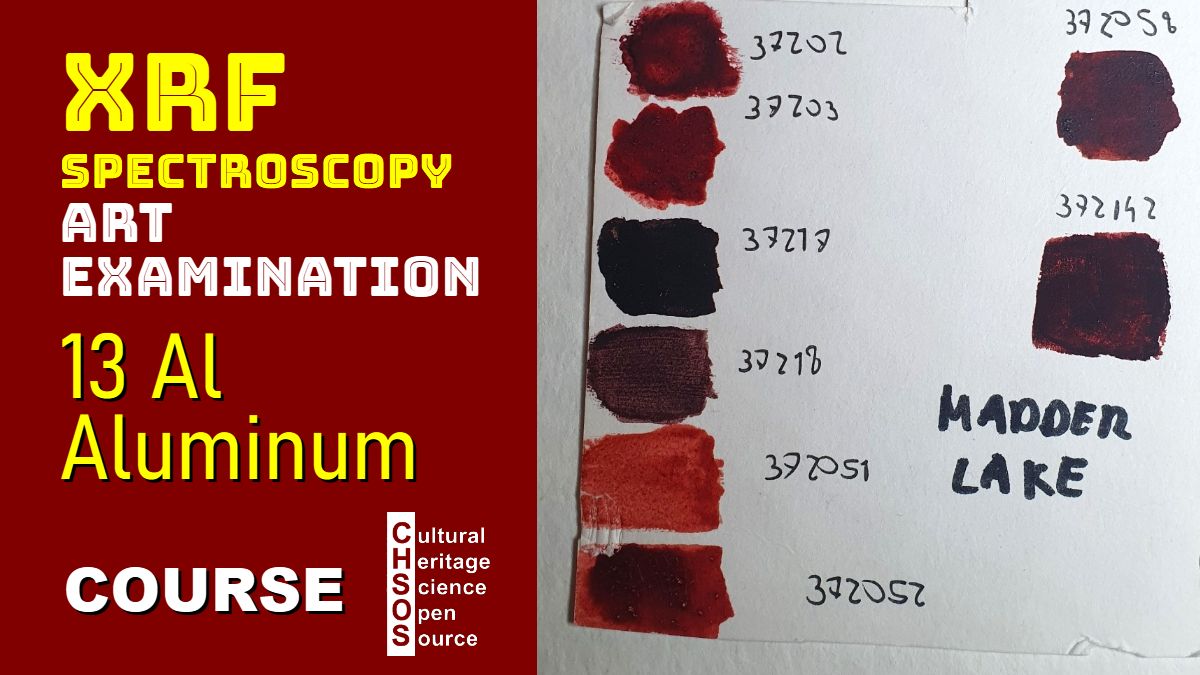
- Various Madder Lake pigment samples
- Introduction to Aluminum Detection
- Explain the K-alpha line of aluminum at 1.5 keV.
- Discuss attenuation effects due to air path and transmission calculations.
- Baseline Measurements
- Analyze pure aluminum and alum samples.
- Madder Lake Analysis
- Introduce the role of mordants in lake pigments formation.
- Examine different Madder Lake samples and their elemental fingerprints.
- Identify other elements (potassium, calcium, tin, iron, phosphorus, sulfur, copper) indicating alternative mordants.









To reinforce the concepts discussed here, we also provide a video lesson that visually walks through the key points of this topic. Watching the video alongside the text can help you better understand and apply these ideas in practice.
The course XRF Spectroscopy for Art Examination introduces conservators, art historians, and scientists with interest in Art to the principles and practical applications of X-ray fluorescence (XRF) spectroscopy in the examination of artworks. The course starts with basic principles of XRF and gradually explores its role in identifying materials and methods used in the creation and conservation of art.
Course Objectives
- Understand the fundamentals of XRF spectroscopy and how it applies to the analysis of art.
- Learn the key features and limitations of XRF for examining art and archaeology.
- Gain skills in interpreting XRF spectra to identify specific elements in paint layers, inks and metals.









