- Understand the process leading to the generation of X-ray emission lines.
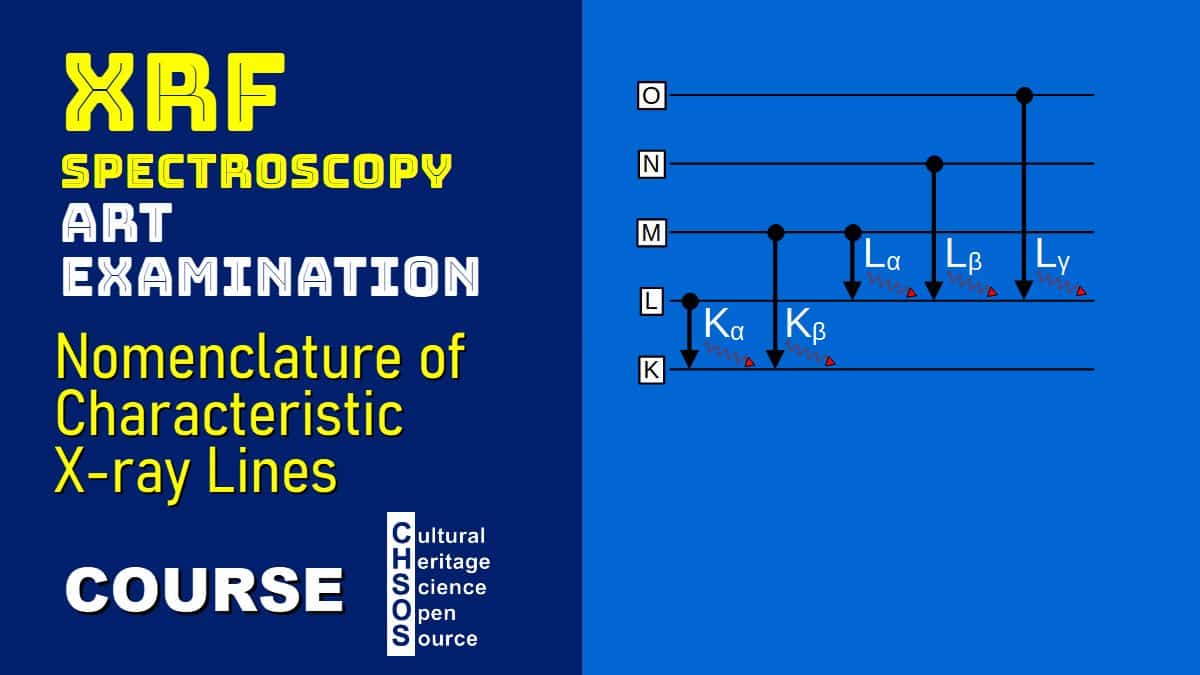
- Learn the nomenclature for X-ray emission lines, such as Kα, Kβ, Lα, and Lβ.
- Explore the relationship between energy transitions and emission lines.
- Use reference tables to identify emission lines in XRF spectra.
- Reference tables for XRF emission lines, including:
- XRF peaks of chemical elements table (art-focused).
- Online resources from Horiba Jobin Yvon and Lawrence Berkeley National Laboratory.
- Introduction to Atomic Structure and X-Ray Emission
- Review the atomic structure and the photoelectric effect as the origin of X-ray emission lines.
- Explain the process of electron transitions between shells (e.g., K, L, M) and the energy release in the form of characteristic X-rays.
- Nomenclature of X-Ray Emission Lines
- Discuss how transitions are named based on the shell where the vacancy is filled (e.g., Kα, Kβ, Lα).
- Highlight the energy differences between lines and their significance.


Free Course: XRF Spectroscopy for Art Examination
The course XRF Spectroscopy for Art Examination introduces conservators, art historians, and scientists with interest in Art to the principles and practical applications of X-ray fluorescence (XRF) spectroscopy in the examination of artworks. The course starts with basic principles of XRF and gradually explores its role in identifying materials and methods used in the creation and conservation of art.
Course Objectives
- Understand the fundamentals of XRF spectroscopy and how it applies to the analysis of art.
- Learn the key features and limitations of XRF for examining art and archaeology.
- Gain skills in interpreting XRF spectra to identify specific elements in paint layers, inks and metals.
Scientific Art Examination – Resources:
Getty Conservation Institute (GCI) – USA
The British Museum – Scientific Research Department – UK
Scientific Research Department – The Metropolitan Museum of Art, New York, USA
C2RMF (Centre de Recherche et de Restauration des Musées de France) – France
Rijksmuseum – Science Department – Netherlands








