Objectives:
- Learn to identify copper presence in various pigments using XRF spectroscopy.
- Gain proficiency in setting up XRF equipment to detect copper in low-concentration pigments.
Materials:
- XRF Spectrometer
- Pigments Checker STANDARD
Lesson Plan: 1. Introduction to Copper in Pigments:
- Discuss the historical use of copper in pigments and its importance in ancient art.
- Introduce modern copper-containing pigments, with an emphasis on phthalocyanine blue and green.
- Explain how copper has a strong XRF signal, but certain pigments with low copper content (e.g., phthalocyanine blue) require adjustments.
- Outline the adjustments in filter setup needed for low-concentration detection, focusing on low-energy peaks between 2-10 keV.
- Sample 1: Malachite
- Position malachite sample for XRF acquisition.
- Analyze the spectrum, highlighting copper’s k-alpha and k-beta peaks at approximately 8 and 8.9 keV.
- Sample 2: Azurite
- Run XRF acquisition on azurite and compare the spectrum with malachite.
- Discuss differences in copper content, noting azurite’s higher copper count and resulting stronger signal.
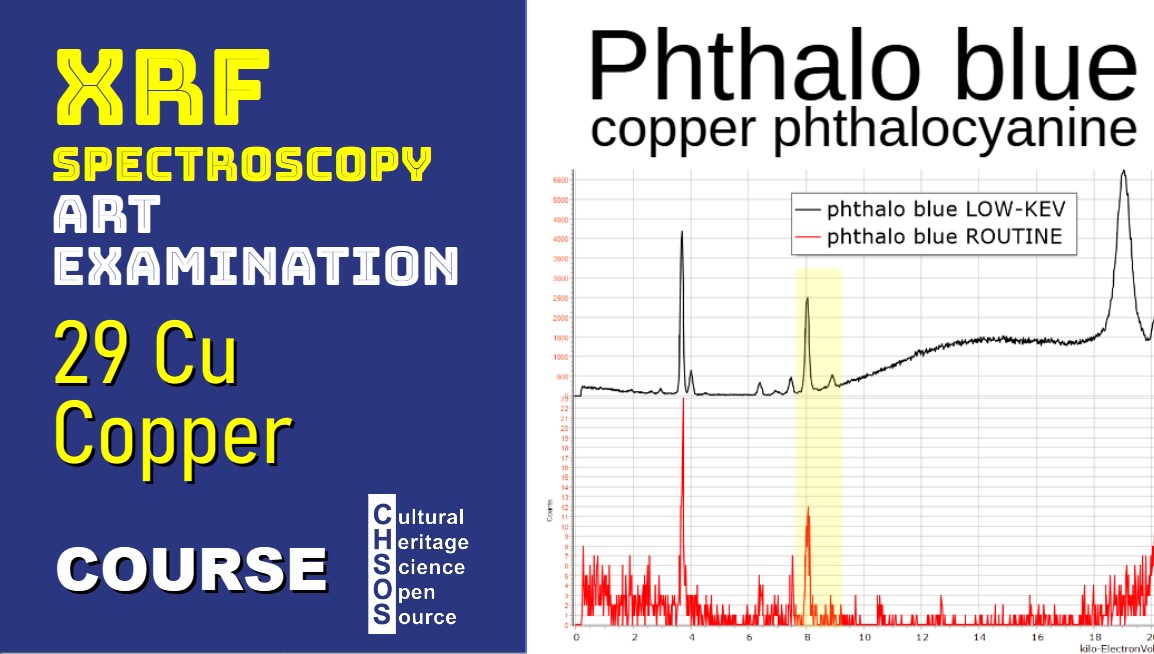
- Sample 3: Phthalocyanine Blue
- Conduct an XRF acquisition on phthalocyanine blue, observing the low copper signal.
- Adjust filters to a 0.25 mm aluminum filter, increasing microampere settings, and examine the resulting spectrum for clearer copper peaks.




Free Course: XRF Spectroscopy for Art Examination
The course XRF Spectroscopy for Art Examination introduces conservators, art historians, and scientists with interest in Art to the principles and practical applications of X-ray fluorescence (XRF) spectroscopy in the examination of artworks. The course starts with basic principles of XRF and gradually explores its role in identifying materials and methods used in the creation and conservation of art.
Course Objectives
- Understand the fundamentals of XRF spectroscopy and how it applies to the analysis of art.
- Learn the key features and limitations of XRF for examining art and archaeology.
- Gain skills in interpreting XRF spectra to identify specific elements in paint layers, inks and metals.
Scientific Art Examination – Resources:
Getty Conservation Institute (GCI) – USA
The British Museum – Scientific Research Department – UK
Scientific Research Department – The Metropolitan Museum of Art, New York, USA
C2RMF (Centre de Recherche et de Restauration des Musées de France) – France
Rijksmuseum – Science Department – Netherlands








